Carolin Pirkl
q3-MuPa: Quick, Quiet, Quantitative Multi-Parametric MRI using Physics-Informed Diffusion Models
Dec 19, 2025Abstract:The 3D fast silent multi-parametric mapping sequence with zero echo time (MuPa-ZTE) is a novel quantitative MRI (qMRI) acquisition that enables nearly silent scanning by using a 3D phyllotaxis sampling scheme. MuPa-ZTE improves patient comfort and motion robustness, and generates quantitative maps of T1, T2, and proton density using the acquired weighted image series. In this work, we propose a diffusion model-based qMRI mapping method that leverages both a deep generative model and physics-based data consistency to further improve the mapping performance. Furthermore, our method enables additional acquisition acceleration, allowing high-quality qMRI mapping from a fourfold-accelerated MuPa-ZTE scan (approximately 1 minute). Specifically, we trained a denoising diffusion probabilistic model (DDPM) to map MuPa-ZTE image series to qMRI maps, and we incorporated the MuPa-ZTE forward signal model as an explicit data consistency (DC) constraint during inference. We compared our mapping method against a baseline dictionary matching approach and a purely data-driven diffusion model. The diffusion models were trained entirely on synthetic data generated from digital brain phantoms, eliminating the need for large real-scan datasets. We evaluated on synthetic data, a NISM/ISMRM phantom, healthy volunteers, and a patient with brain metastases. The results demonstrated that our method produces 3D qMRI maps with high accuracy, reduced noise and better preservation of structural details. Notably, it generalised well to real scans despite training on synthetic data alone. The combination of the MuPa-ZTE acquisition and our physics-informed diffusion model is termed q3-MuPa, a quick, quiet, and quantitative multi-parametric mapping framework, and our findings highlight its strong clinical potential.
Region of Interest focused MRI to Synthetic CT Translation using Regression and Classification Multi-task Network
Mar 30, 2022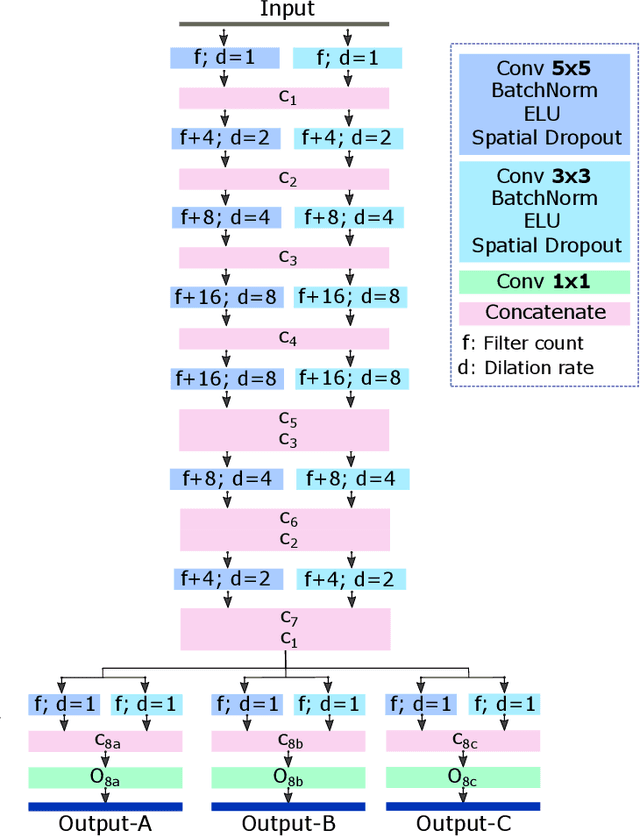
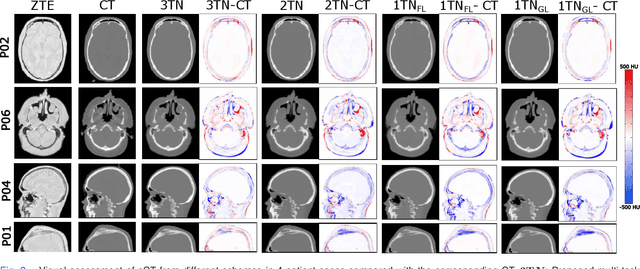
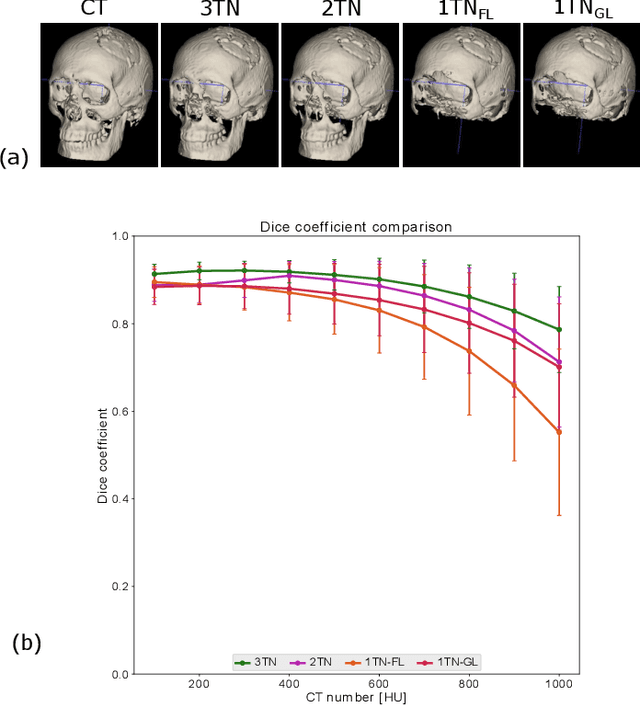
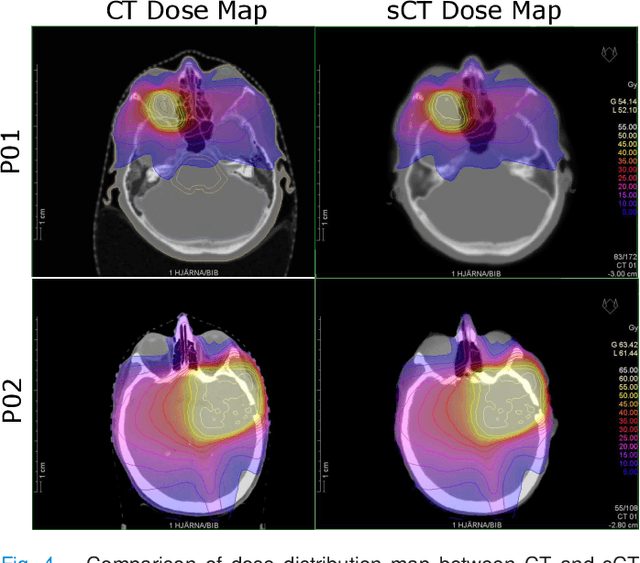
Abstract:In this work, we present a method for synthetic CT (sCT) generation from zero-echo-time (ZTE) MRI aimed at structural and quantitative accuracies of the image, with a particular focus on the accurate bone density value prediction. We propose a loss function that favors a spatially sparse region in the image. We harness the ability of a multi-task network to produce correlated outputs as a framework to enable localisation of region of interest (RoI) via classification, emphasize regression of values within RoI and still retain the overall accuracy via global regression. The network is optimized by a composite loss function that combines a dedicated loss from each task. We demonstrate how the multi-task network with RoI focused loss offers an advantage over other configurations of the network to achieve higher accuracy of performance. This is relevant to sCT where failure to accurately estimate high Hounsfield Unit values of bone could lead to impaired accuracy in clinical applications. We compare the dose calculation maps from the proposed sCT and the real CT in a radiation therapy treatment planning setup.
Compressive MRI quantification using convex spatiotemporal priors and deep auto-encoders
Jan 23, 2020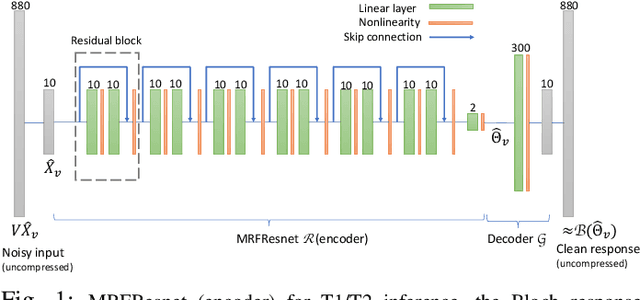
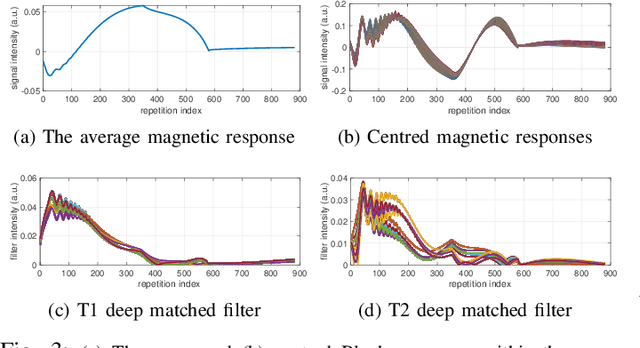
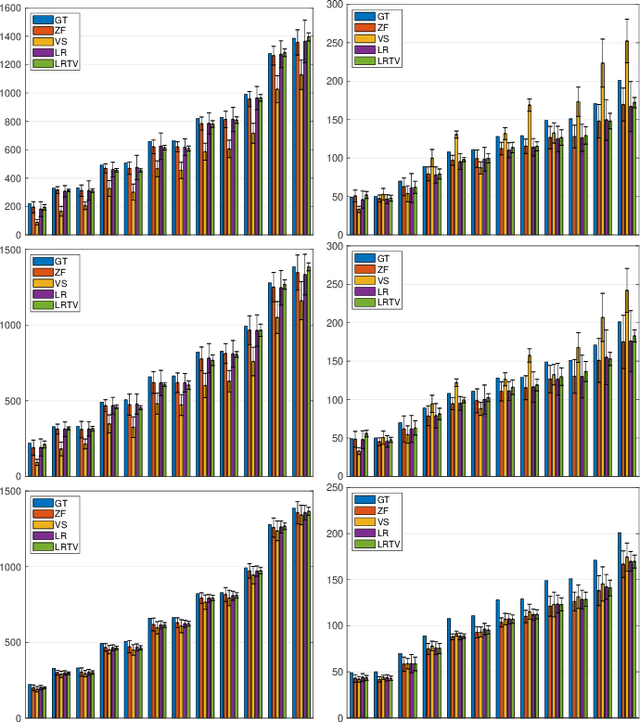
Abstract:We propose a dictionary-matching-free pipeline for multi-parametric quantitative MRI image computing. Our approach has two stages based on compressed sensing reconstruction and deep learned quantitative inference. The reconstruction phase is convex and incorporates efficient spatiotemporal regularisations within an accelerated iterative shrinkage algorithm. This minimises the under-sampling (aliasing) artefacts from aggressively short scan times. The learned quantitative inference phase is purely trained on physical simulations (Bloch equations) that are flexible for producing rich training samples. We propose a deep and compact auto-encoder network with residual blocks in order to embed Bloch manifold projections through multiscale piecewise affine approximations, and to replace the nonscalable dictionary-matching baseline. Tested on a number of datasets we demonstrate effectiveness of the proposed scheme for recovering accurate and consistent quantitative information from novel and aggressively subsampled 2D/3D quantitative MRI acquisition protocols.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge